





Published on Nov 30, 2023
Polymer light emitting diodes (PLEDs) are optoelectronic devices based on small molecule or polymers that emit light when an electric current flow through them. A simple PLED consist of a fluorescent polymer layer sandwiched between two metal electrodes. Under application of an electric field electrons and holes are injected from the two electrodes in to the polymer layer, where they meet and recombine to produce light.
They have been developed for application in flat panel displays. Envision light weight, portable and flexible flat panel displays that provide visual imagery that is easy to read, vibrant in colour and less consuming of power.
PLEDs are light weight, durable power efficient and ideal for portable application. PLEDs fewer process steps and also use both fewer low cost materials than LCD displays. PLEDs can replace the current technology in many applications due to following performance advantages over LCD s
*Greater brightness
*Faster response time for full motion video
*Full viewing angle
*Lighter weight
*Greater environmental durability
*More power efficiency
*Broader operating temperature range
*Greater cost effectiveness
Light Emitting Polymers (LEPs) are semi-conducting devices that exhibit electroluminescent characteristics. The phenomenon known as electroluminescence (EL) is caused by the emission of light generated from the recombination of electrons and holes electrically injected into a semi-conductor. So far conventional and marketable electroluminescent devices (ED s) have always been inorganic semi-conductors but recent developments in the late 1990s are opening the doors for organic materials. Obtaining organic materials with electroluminescent properties was first achieved in the 1960s on anthracene crystals by Pope et al at New York University.
However these early devices had high operating voltages and very low quantum efficiency thus they did not attract much attention for two decades. In 1987 a breakthrough was made by Tang and VanSlyke at Eastman Kodak who, by using multi layers of sublimated organic molecules, succeeded in reducing the operating voltage dramatically and increasing the quantum efficiency significantly. Another important discovery was made in 1990 by Burroughs Et al at Cambridge University who detected electroluminescence from diodes based on luminescent conjugated polymers.
Polymer means many parts Polymers are very large organic molecules formed by thousands of repeating units linked together. One can view these heavy organic molecules (molecular weight between 5,000 and 150,000) as very long chains mostly made of C-C, C-O and C-N bonds. Polymer technology has existed for decades.
Use of natural rubber and common plastics such as polythene dates back to as early as 1820s. However finding polymers with intrinsic properties of semi-conductors is very recent. In 1967, electrically conducting polymers from pyrrole, thiopene and furan were characterized and the electrical conductivity of poly(anilines) noted. The report in 1977about doping polyacetylene to achieve conductivity opened up important vistas for chemistry and physics.
Polymer based light-emitting diodes (LEDs) were discovered in 1990 by Friend et al. The first polymer LED used 'poly phenylene vinylene' (PPV) as the emitting layer. Since 1990 many different polymers have been shown to emit light under the application of an electric field (EL). PPV and its derivatives are still the most commonly used materials, but polythiophenes polyphenylenes, and polypyridines are now being tested for higher efficiency, longer lifetime and lower power requirements.
A typical polymer LED consists of a thin film of a luminescent conjugated polymer sandwiched between an anode and a cathode, on top of a glass substrate. Indium Tin Oxide (ITO) is most frequently used as the anode because it exhibits conductive properties with a relatively high work function. It also is optically transparent, thus allowing the light to propagate through the device. The counter electrode (cathode) is usually a metal with low work function, enabling the electron injection into the conduction band of the polymer medium. Calcium, aluminum, Al/lithium as well as magnesium/silver alloys are generally used
A layer of conducting polymer between the indium–tin oxide and the light-emitting polymer helps to improve the injection of holes. (The extra layer is deliberately kept thin so that the device remains transparent.) One good example is poly (ethylene dioxy) thiophene, or “PEDOT”, a material that was developed by Bayer, the German chemical company. PEDOT can be mixed with poly (styrene sulphonic acid), or “PSS”, to form a stable and highly conducting charge-transfer complex, and this can be deposited from an aqueous solution to form a layer on the indium–tin oxide .The PEDOT/PSS layer has a high work function which allows holes to be injected easily into the PPV since the barrier is now much smaller.
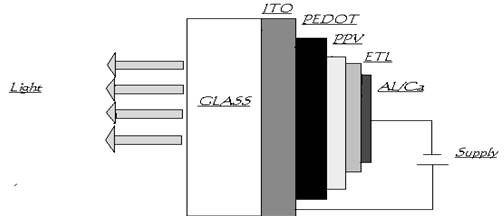
Another advantage of the PEDOT/PSS layer is that it smoothes out the relatively rough surface of the indium–tin oxide, thereby preventing any local short-circuiting that would otherwise cause the device to fail.
There is another layer called electron transport layer (ETL) which is present between the cathode and PPV. It allows electrons to be injected easily in to the PPV layer.
PLEDs are grown on a glass or plastic substrate to form a multi layer structure about 100nm thick. The substrate is first coated with a conducting transparent electrode such as indium tin oxide (ITO) or poly aniline which serves as anode. This is followed by a thin hole transporting organic layer called PEDOT layer (poly ethylene dioxy thiophene)
A polymer light emitting layer of comparable thickness is the deposited on to the PEDOT surface. The later stratum often doubles as the electron transporting layer (ETL). Finally the devise is completed by depositing a cathode consisting of a metal with a low work function such as calcium or alloy such as magnesium silver on to the ETL surface. A low work function is necessary to ensure efficient low resistance injection of electrons from the cathode in to the ETL.
Changing the composition of the layers tunes the PLED emission colour across the visible spectrum. Green emission can be achieved by dopping an electron conducting polymer matrix called Alq3 with either a small amount of iridium phosphor or fluorescent dyes. The pigment perylene when doped into an ETL known as CBP emits blue light. Lanthanide complexes and porphyrin pigments have been used to efficiently emit red light when doped in to Alq3 or CBP.
PPV (Poly phenylene vinylene) has a fully conjugated backbone (figure 2), as a consequence the HOMO of the macromolecule stretches across the entire chain, this kind of situation is ideal for the transport of charge; in simple terms, electrons can simply "hop" from one π orbital to the next since they are all linked.
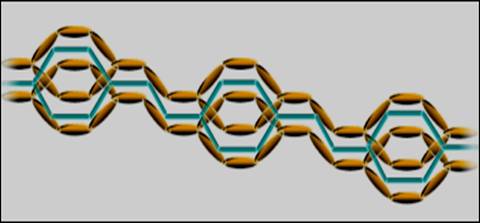
Fig2: A demonstration of the full conjugation of π electrons in PPV.
PPV is a semiconductor. Semiconductors are so called because they have conductivity that is midway between that of a conductor and an insulator. While conductors such as copper conduct electricity with little to no energy (in this case potential difference or voltage) required to "kick-start" a current, insulators such as glass require huge amounts of energy to conduct a current .Semiconductors require modest amounts of energy in order to carry a current, and are used in technologies such as transistors, microchips and LED s.
| Are you interested in this topic.Then mail to us immediately to get the full report.
email :- contactv2@gmail.com |